CME: Diagnosis and Treatment of Four Mosquito-Borne Viral Illnesses
- FibonacciMD

- Jun 24, 2021
- 16 min read
Updated: Jun 24, 2025
Zika, Dengue, Chikungunya, and West Nile
Infectious Disease and Emergency Medicine
✅ Earn Free CME Credit for Reading This Article
Eligible for 0.75 PRA Category 1 Credit
Click the button below to take a short quiz.
A valid email is required to send your certificate.
We’ll send you occasional updates. Your email stays private—never sold or shared. 😇

No other animal infects and causes disease as effectively as mosquitos, which are the deadliest animals in the world to humans. Infections caused by mosquitos kill over 700,000 people a year.[2] In this article we will look at four mosquito borne viral diseases; Zika, dengue, chikungunya, and West Nile. Currently West Nile is endemic in the continental US, but clinicians may see the other three diseases in travelers who are either from, or who have visited endemic areas. It is also possible that like Zika a few years ago, these diseases may become endemic in the U.S. at some time in the future.
Zika Virus
In 2015 and 2016 Zika was a front-page news story due to surging infections in the Americas, with the threat of severe fetal brain defects occurring if pregnant women were infected. Since then, Zika has almost disappeared.[3] In 2016, there were 5,168 reported cases in the U.S. and 36,512 cases in U.S. territories, with widespread transmission in Puerto Rico and the U.S. Virgin Islands, and limited local transmission in Florida and Texas.[4] Brazil had 200,000 reported Zika cases in 2016, and infants born with microcephaly increased 10-fold from prior levels in late 2015 to early 2016.[5] By 2019, there were only 27 reported U.S. cases in travelers and 74 cases in U.S. territories.[6] There are currently no countries with a large outbreak of Zika. Some experts do not think pesticide spraying has caused the decline, and it is possible that a form of herd immunity in endemic areas has occurred, limiting the ability of mosquitos to spread Zika to humans.[3]
Zika virus is an RNA Flavivirus transmitted by Aedes aegypti and Aedes albopictus mosquitos. Human to human transmission also occurs, and potentially it can be transmitted by blood transfusion and sexual contact. Zika virus was first discovered in 1947 and is named after the Zika Forest in Uganda. The first human case was reported in 1952.[7]
Zika Clinical Disease[8]

Most people infected with Zika virus are asymptomatic. Clinical findings typically are acute onset of fever with maculopapular rash, arthralgia, and/or conjunctivitis. Myalgias and headaches are common. The illness is usually mild with symptoms lasting from several days to a week. Severe disease requiring hospitalization is uncommon, and fatalities are rare. There have been some cases of Guillain-Barré syndrome reported after a Zika infection. Zika virus infection during pregnancy can cause fetal microcephaly, decreased brain tissue, and damage to the back of the eyes.[9]
Treatment
No specific treatment is available for Zika virus disease, other than supportive care. As dengue presents similarly, and in the same geographic areas as Zika, aspirin and other non-steroidal anti-inflammatory drugs(NSAIDs) should be avoided until dengue can be ruled out, to reduce the risk of hemorrhage. People infected with Zika, should be protected from mosquito exposure during the first few days of illness to reduce the risk of further human to mosquito to human transmission. Sexual relations should be curtailed to prevent passing on the infection.[8]
A vaccine has been developed, but gaining approval by testing it in an environment of decreasing cases has proved difficult.[3]
Sexual Transmission[11]
Zika can be sexually transmitted by sharing sex toys, and by vaginal, anal, and possibly oral sex, even if asymptomatic. Zika virus has been detected in semen, vaginal fluids, saliva, urine, and breast milk. There is no evidence however, that Zika can be transmitted through saliva during kissing. Zika virus has been reported to persist in semen for up to 69 days. It is recommended that pregnant women with sexual partners who live in, or have traveled to, an endemic Zika area, use condoms during sex, or abstain from sexual relations for the duration of the pregnancy. Condom use is recommended after travelling to an endemic area for two months for nonpregnant females, and three months for males, due to the longer survival time in semen.
Testing[12]
Zika testing of symptomatic male and female non-pregnant patients is not currently recommended by the CDC based on current epidemiology, but this guidance may change in the case of a renewed outbreak of cases. However, these patients should be tested for dengue virus, which is more common, and has similar presenting features. Testing asymptomatic males and non-pregnant females for either dengue or Zika viruses is not currently recommended.
Zika testing involves a very complex algorithm for pregnant women. Recommended testing is different if the woman is symptomatic, asymptomatic, has had an abnormal fetal ultrasound consistent with a congenital Zika infection, or has had sexual relations with someone suspected of having the disease.
If you are interested in learning more about the complete CDC Zika testing guidelines algorithm for pregnant women, follow this link: https://www.cdc.gov/zika/hc-providers/testing-guidance.html
Dengue
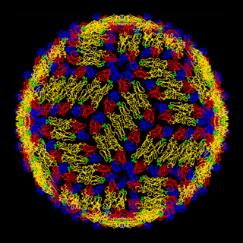
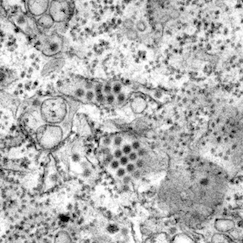
Structure of a dengue virus[13] Dengue viruses in tissue[14]
Dengue viruses are Flaviviruses, transmitted through the bite of infected Aedes aegypti or Aedes albopictus mosquitos. Dengue is caused by one of four related viruses: Dengue virus 1, 2, 3, and 4. For this reason, a person can be infected as many as four times with the different dengue viruses.[15]
The history of dengue is not well known, but a dengue-like outbreak in humans was recorded in a Chinese medical encyclopedia in 992. In the 1700s dengue was known as breakbone fever. Queen Luisa of Spain used the word dengue while writing about her recovery from it in 1801. No one is sure about dengue’s etymology, but the word dengue in Spanish means affectation, or careful, and may have described the stiff, painful movements of people with dengue fever. Another theory is that the name came from a Swahili phrase “Ka dinga pepo”, or “disease caused by an evil spirit”.[16]
Dengue is common in more than 100 countries around the world. About three billion people live in endemic areas, and every year up to 400 million people get infected with dengue, approximately 100 million people get symptomatic illness, and 22,000 die from severe dengue.[15]
Dengue is common in the U.S. territories of Puerto Rico, the U.S. Virgin Islands, and American Samoa. Nearly all dengue cases reported in the U.S. mainland are from travelers infected elsewhere. In 2019, there were 1,203 cases of dengue reported in the U.S., and 56 cases reported in U.S. territories.[17] Dengue is a frequent cause of infection in Central and South America, East Africa, Southeast Asia and the Pacific Islands.[18]

World Health Organization(WHO) Clinical Dengue Definitions[19]
Dengue is defined by a combination of ≥2 clinical findings in a febrile person who traveled to, or lives in a dengue-endemic area. Clinical findings include nausea, vomiting, rash, aches and pains, a positive tourniquet test, leukopenia, or the following warning signs which may predict severe dengue: abdominal pain or tenderness, persistent vomiting, clinical fluid accumulation, mucosal bleeding, lethargy, restlessness, and liver enlargement.
Severe dengue is defined as dengue with any of the following symptoms: severe plasma leakage leading to shock or fluid accumulation with respiratory distress; severe bleeding; or severe organ impairment such as elevated transaminases ≥1,000 IU/L, impaired consciousness, or heart impairment.
Dengue Clinical Disease and Treatment[19]
A study of antibody dependent enhancement of dengue in children found that the level of serum antibodies from a first infection determined the risk of severe dengue disease on reinfection. The presence of high dengue antibody titers in an individual are protective, but people with intermediate antibody titers had a much higher likelihood of developing severe dengue, even greater than those with low antibody titers. In that study the chances of getting severe dengue during a second infection was 1.6% in the high antibody titer group, 11.4% in the intermediate antibody titer group, and 6.6% in low antibody titer group. It is thought that particularly with intermediate dengue antibody titers, some binding of antibodies to the dengue virus occurs, but the antibody does not necessarily neutralize the virus. This creates a virus-antibody complex that appears to facilitate viral entry into host cells, and can trigger an immune cascade that leads to severe dengue. This is felt to be the reason why a dengue re-infection with a different strain, or first infection after dengue vaccination, may increase the risk of contracting severe dengue.[20

Dengue begins abruptly after a typical incubation period of five to seven days, and has three phases: febrile, critical, and convalescent or recovery.
Febrile Phase
The febrile phase lasts two to seven days and can be biphasic. Other signs and symptoms may include severe headache, retro-orbital eye pain, muscle, joint, bone pain, and/or a macular or maculopapular rash. Some patients have an injected oropharynx and facial erythema the first 24–48 hours after onset. Bleeding may occur from thrombocytopenia, or in more ill patients from a coagulopathy.
Thrombocytopenia results from transient bone marrow suppression, and increased peripheral destruction of platelets in dengue.[22] In this phase, patients may have minor hemorrhagic manifestations such as ecchymosis, purpura, epistaxis, bleeding gums, or hematuria. Petechiae may occur, and a positive tourniquet test may aid in the diagnosis of dengue. A positive tourniquet test result is ten or more petechiae per one square inch in the arm, after inflating a blood pressure cuff halfway between the systolic and diastolic pressures for two minutes.[23]

Critical Phase
The critical phase of dengue begins at defervescence, and typically lasts 24–48 hours. Most patients clinically improve during this phase, but there can be substantial plasma leakage due to a marked increase in vascular permeability. Patients with severe plasma leakage and third spacing may have pleural effusions, ascites, hypoproteinemia, or hemoconcentration. Liver enlargement may occur.[22] Once hypotension develops, irreversible shock and death may ensue despite resuscitation. Patients can also develop severe hemorrhagic manifestations such as hematemesis, bloody stools, or menorrhagia. Increased activated partial thromboplastin time (APTT), and a reduction in fibrinogen levels may be seen in severe dengue.[22] Uncommon complications include myocarditis, pancreatitis, and encephalitis.
Convalescent/Recovery Phase[19]
In severe dengue, as plasma leakage and third spacing subsides, the patient enters the convalescent or recovery phase, and begins to reabsorb extravasated intravenous fluids, pleural effusions and ascites. As a patient’s hemodynamic status stabilizes there is a significant diuresis of the excess extracellular fluid. The patient’s hematocrit stabilizes, or may fall because of the dilutional effect of the reabsorbed fluid, and the white cell count usually starts to rise, followed by a recovery of platelet count. In the convalescent-phase rashes may desquamate and become pruritic.
Dengue and Pregnancy[15]
There is limited data about dengue during pregnancy. Perinatal transmission can occur, and maternal infection may increase the likelihood of symptomatic infection in the newborn. Of 41 perinatal transmission cases to fetuses described in the literature, all developed thrombocytopenia, most had evidence of plasma leakage typically with ascites or pleural effusions, and 39 were febrile. Nearly 40% had a hemorrhagic manifestation, and 25% were hypotensive at some point. Perinatally infected neonates typically become ill during the first week of life. Placental transfer of maternal dengue IgG antibodies from a previous infection does occur, but when the protective effect of these antibodies wanes, infants 6–12 months of age are at risk for severe dengue.
Laboratory Findings and Testing[24]

Laboratory findings commonly include leukopenia, thrombocytopenia, hyponatremia, elevated aspartate aminotransferase and alanine aminotransferase, and in the majority of cases, a normal erythrocyte sedimentation rate.[26]
During days one to seven after symptom onset, dengue virus RNA can be detected with molecular tests, such as an RT-PCR(reverse transcriptase polymerase chain reaction). NS1 is a dengue virus protein that also can be detected by some commercial tests. An IgM antibody level should also be drawn. A negative result from a molecular, NS1, or IgM antibody test is not conclusive. After seven days post symptom onset, patients with initially negative RT-PCR, NS1,and IgM antibody tests from the first seven days of illness should have a convalescent sample tested for IgM antibodies. During the convalescent phase, IgM antibodies are usually present and can be reliably detected. IgM antibodies against dengue virus can remain detectable for 3 months or longer after infection.
If a PCR or NS1 test is positive for dengue, a current dengue diagnosis is confirmed. If the PCR result is negative and the IgM antibody test is positive, the laboratory diagnosis is presumptive dengue virus infection.
Cross reactivity with other flaviviruses such West Nile, yellow fever, and Zika, is a limitation of dengue IgM antibody tests. Therefore, a patient with past flavivirus infection(s) may be falsely test positive for dengue virus IgM antibodies. To determine if dengue is causing the infection, IgM positive specimens should be tested for specific neutralizing antibodies by a plaque reduction neutralization test (PRNT).
Whenever a pregnant woman is tested for dengue, it is recommended Zika also be tested for using an RT-PCR test, as they may present similarly.
Treatment[27]
No specific antiviral agents or treatments exist for dengue. Supportive care is recommended and patients should try to stay well hydrated, and avoid aspirin and other NSAIDS because of their anticoagulant properties. Fever can be controlled with acetaminophen and possibly tepid sponge baths. Febrile patients should avoid mosquito bites to reduce risk of further transmission to other people.
In severe dengue, ICU care may be required. Prophylactic platelet transfusions in dengue patients are not beneficial, and may contribute to fluid overload. Administration of corticosteroids has not demonstrated any benefit, except in the case of autoimmune-related complication such as immune thrombocytopenia purpura.
A vaccine to prevent dengue, Dengvaxia, administered in three doses six months apart, is licensed and available in some countries for people aged 9-45 years old. The WHO recommends that the vaccine only be given to people with confirmed prior dengue virus infection, as previously uninfected people who are vaccinated and then get dengue have a higher chance of developing severe dengue. In 2019, Dengvaxia was FDA approved for use in children 9-16 years old, with laboratory confirmed prior dengue virus infection, living in an endemic area such as the U.S. territories of American Samoa, Guam, Puerto Rico or the U.S. Virgin Islands.[28]
Chikungunya Virus
Chikungunya is a mosquito-borne viral disease first described during an outbreak in southern Tanzania in 1952. The name chikungunya comes from the Tanzanian Kimakonde language, and means to become contorted, due to the stooped appearance of sufferers with severe joint pains.[29] It is an RNA virus that belongs to the alphavirus genus of the family Togaviridae. It is most commonly spread by Aedes aegypti and Aedes albopictus mosquitos, although blood-borne transmission is possible. Rare in utero transmission has been documented, but it is not transmitted by breast milk.[30]
Chikungunya virus cases and outbreaks have been identified in countries in Africa, Asia, Europe, and the Indian and Pacific Oceans. In late 2013, the first local transmission of Chikungunya in the Americas was identified in the Caribbean, and the virus then spread throughout much of the Americas. During epidemics around the world, hundreds of thousands of cases can occur.[29,31] In 2019, there were 192 reported cases of Chikungunya in the U.S., all from travel, and 2 cases in U.S. territories thought to be from local spread.[32]

Clinical Course and Symptoms[34]
Chikungunya virus infection should be considered in patients with acute onset of fever and polyarthralgia, especially travelers who recently returned from endemic areas.[26]
The majority of people infected with chikungunya virus become symptomatic. The incubation period is typically three to seven days with a range of one to twelve days. Symptoms include acute onset of fever (typically >39°C) and polyarthralgia. Joint symptoms are usually bilateral, symmetric, and can be severe and debilitating. Other symptoms can include headache, myalgia, arthritis, conjunctivitis, nausea/vomiting, or maculopapular rash.
Clinical laboratory findings can include lymphopenia, thrombocytopenia, elevated creatinine, and elevated hepatic transaminases.
Acute symptoms typically resolve within seven to ten days. Rare complications include uveitis, retinitis, myocarditis, hepatitis, nephritis, bullous skin lesions, hemorrhage, meningoencephalitis, myelitis, Guillain-Barré syndrome, and cranial nerve palsies. People at risk for severe disease include neonates exposed intrapartum, the elderly, and people with previous underlying medical conditions. Patients may have a relapse of rheumatologic symptoms such as polyarthralgia, polyarthritis, or tenosynovitis in the months following an acute illness. Persistent joint pains for some can last from months to years. Mortality is rare, and occurs mostly in the elderly.

Diagnostic Testing
Viral culture may detect the virus in the first three days of illness. During the first eight days of illness, chikungunya viral RNA by RT-PCR will often be positive. Chikungunya virus antibodies normally start to develop toward the end of the first week of illness. Acute phase samples may be positive for IgM, although IgG should also be tested for. Convalescent samples should be obtained from patients whose acute-phase samples are negative, with both IgG and IgM antibodies tested.[36,37]
Treatment[34,37,38]
There is no vaccine to prevent chikungunya virus, and treatment is symptomatic. It is recommended that for the first week of infection the patient avoid mosquito bites, as it may be passed on to another person that way. It is difficult to distinguish chikungunya and dengue based on clinical findings alone due to the fact that chikungunya and dengue viruses are transmitted by the same mosquitoes, can circulate in the same area, and occasionally cause co-infections in the same patient. Patients with suspected chikungunya should be managed as dengue until dengue has been ruled out, as proper clinical management of dengue reduces the risk of medical complications and death. It is recommended not to administer aspirin and other NSAIDS until dengue is ruled out, the patient has been afebrile ≥48 hours, and has no warning signs of severe dengue, to reduce the risk of bleeding. Warning signs for severe dengue include severe abdominal pain, persistent vomiting, mucosal bleeding, pleural effusion, ascites, lethargy, enlarged liver, and increased hematocrit with thrombocytopenia.
Persistent joint pain from a chikungunya infection, after dengue has been ruled out, may benefit from use of NSAIDs, corticosteroids, or physiotherapy.
Differentiation between Chikungunya and Dengue[38]
Chikungunya is more likely to cause high fever, severe polyarthralgia, arthritis, rash, and lymphopenia, and dengue virus is more likely to cause neutropenia, thrombocytopenia, hemorrhage, shock, and death. The table below highlights the differences between these two disease entities.

West Nile Virus
West Nile virus (WNV), is a Flavivirus, and the leading cause of mosquito-borne disease in the continental U.S.[39] WNV was first isolated in a woman in the West Nile district of Uganda in 1937, which is how it got its name.[40] It is most often spread in the summer and fall by the bite of an infected mosquito. Infections have rarely occurred through organ transplant, blood transfusions and breast milk.[38] Most people infected with WNV are asymptomatic, but about 20% of those who are infected develop a fever and/or other symptoms. Only about 0.7% of infections lead to a serious and sometimes fatal illness.[39]
Mosquitoes of the genus Culex are generally considered the principal vectors of WNV, in particular Culex Pipiens. Birds are the reservoir hosts of WNV. Interestingly, in Europe, Africa, Middle East and Asia, mortality in birds with WNV infection is rare. However, the virus is highly toxic to birds in the Americas.[40] Dying birds may be an indication of a WNV epidemic in an area. Horses may also become infected.
In 2020, 44 states reported WNV infections in people, birds, or mosquitoes. There were 422 reported human cases of neuroinvasive disease(such as meningitis or encephalitis) and 135 cases of non-neuroinvasive disease.[41] In some previous years there were many more reported cases, with 2,647 cases reported in 2018, and 5,674 cases in 2012.[42]

Clinical Presentation and Symptoms[44]
WNV should be considered in anyone in an endemic area presenting with a febrile, or acute neurologic illness, who has had recent exposure to mosquitoes, blood transfusion, or organ transplantation, especially during the summer months. The diagnosis should also be considered in infants whose mother was infected with WNV during pregnancy or while breastfeeding.
The incubation period for WNV disease is typically two to six days but ranges from two to 14 days, and can be longer, up to several weeks, in immunocompromised people.
It is estimated that 70-80% of human WNV infections are subclinical or asymptomatic. Symptomatic WNV presents with an acute, non-specific febrile illness that typically includes headache, weakness, myalgia, arthralgia, gastrointestinal symptoms, and a transient maculopapular rash.
About 0.7% of infected persons develop neuroinvasive disease, which typically manifests as meningitis, encephalitis, or acute flaccid paralysis. WNV meningitis is indistinguishable from viral meningitis due to other etiologies, and typically presents with fever, headache, and nuchal rigidity. WNV encephalitis is more severe and presents with fever, altered mental status, seizures, focal neurologic deficits, or movement disorders such as tremor or parkinsonism. WNV acute flaccid paralysis, also known as WNV poliomyelitis, is clinically and pathologically identical to poliomyelitis, with damage to spinal anterior horn cells that may progress to respiratory paralysis requiring mechanical ventilation. WNV flaccid paralysis often presents as an isolated limb paresis or paralysis, and can occur without fever or viral prodrome. WNV-associated Guillain-Barré syndrome and WNV radiculopathy have also been reported, and can be distinguished from WNV poliomyelitis by clinical manifestations, and electrophysiologic testing.
Rarely, cardiac dysrhythmias, myocarditis, rhabdomyolysis, optic neuritis, uveitis, chorioretinitis, orchitis, pancreatitis, and hepatitis have been described in patients with WNV disease.
Most patients with non-neuroinvasive WNV disease or WNV meningitis recover completely, but fatigue, malaise, and weakness can linger for weeks or months. Patients who recover from WNV encephalitis or poliomyelitis often have residual neurologic deficits. The mortality rate for neuroinvasive disease is approximately 10%, but is higher for patients with WNV encephalitis and poliomyelitis than with WNV meningitis.
One study found that five of 25 patients who had WNV infections diagnosed 1.6 to 6.7 years previously continued to have WNV RNA in their urine, suggesting chronic infection. But, according to the CDC, the implications of this are still unknown.[45,46]
Diagnostic Testing[47]
Routine clinical laboratory studies for WNV are generally nonspecific. In patients with neuroinvasive disease, cerebrospinal fluid(CSF) examination generally shows lymphocytic pleocytosis, but neutrophils may predominate early in the course of the illness. Brain MRI is frequently normal, but signal abnormalities in the basal ganglia, thalamus, and brainstem may be seen in patients with encephalitis, and in the anterior spinal cord in patients with WNV poliomyelitis.[44]
Laboratory diagnosis is typically made by testing of serum or CSF for WNV-specific IgM antibodies which are usually detectable three to eight days after the onset of illness, and can persist for 30 to 90 days. The absence of WNV IgM antibodies on an initial sample does not rule out the diagnosis of WNV infection, and a convalescent sample may need to be drawn. Unfortunately, cross-reactive antibodies from other flaviviruses are common, and all positive IgM results should be confirmed with a plaque-reduction
neutralization test (PRNT), which requires acute and convalescent serum, and can differentiate which Flavivirus species is causing the infection.
Viral cultures and RT-PCR testing can be performed on serum, CSF, and tissue specimens that are collected early in the course of illness, and can confirm an infection. Immunohistochemistry can detect WNV antigen in formalin-fixed tissue. Initial negative results of these tests do not rule out a WNV infection.
Treatment[48]
There are no specific treatments for WNV disease, other than supportive management. Patients with meningitis may require pain control for headaches, antiemetics and IV rehydration as needed for nausea and vomiting. Patients with encephalitis require ICU monitoring for the development of elevated intracranial pressure and seizures. WNV encephalitis or poliomyelitis patients should be monitored for the ability to protect their airway from aspiration. Acute neuromuscular respiratory failure may develop in WNV poliomyelitis requiring prolonged ventilatory support.
No WNV vaccines are currently available. Prevention of WNV disease depends on community mosquito control programs, personal protective measures to decrease exposure to infected mosquitoes, and screening of blood and organ donors.
Conclusion
All four of these mosquito-borne viral diseases can present initially as a non-specific illness, making diagnosis difficult. Laboratory testing may help, but initially can be negative, requiring convalescent testing to make a firm diagnosis. Dengue is the deadliest, and in endemic areas initially treating for dengue is recommended until an alternate diagnosis is confirmed. West Nile virus is the only one of the four infections discussed in this article which is currently endemic in the continental U.S., the others are typically seen in travelers who are either from, or who have visited endemic areas. Fortunately, with its threat of fetal abnormalities, Zika virus spread has significantly declined, but around the world dengue and chikungunya infections remain significant health issues.
The infections discussed in this article are just a small portion of the infections that mosquitos can transmit, which include both viruses and parasites, and are listed below.
Mosquito-borne Viruses[49]
· Cache Valley
· Chikungunya
· Dengue
· Eastern equine encephalitis
· Jamestown Canyon
· Japanese encephalitis
· La Crosse encephalitis
· Rift Valley fever
· Ross River virus disease
· St. Louis encephalitis
· West Nile
· Yellow fever
· Zika
Mosquito Borne-Parasites
· Dirofilariasis (dog heartworm)
· Lymphatic filariasis
· Malaria
🎓 Want Free CME Credit for This Article?
Take the quiz now at www.FibonacciMD.app. It only takes a few minutes!
Your certificate will be emailed to you after you pass the quiz and complete a short evaluation
We’ll send you occasional updates. Your email stays private—never sold or shared. 😇